What is happening in my Etchant? ..and what should
My etchant of choice for PCB production is sodium persulfate (Na2S2O8). Chemists call it sodium peroxosulphate, short form NaPS, sodium persulfate is its trade name, the salt of peroxodisulphuric acid. It is a strong oxidizer. It is a severe irritant of the skin, eyes, and respiratory system. It is almost non-hygroscopic and has particularly good ability to be stored for long time. It is easy and safe to handle. It is not combustible, but releases oxygen easily and assists combustion of other materials. (Wikipedia)
Since copper is quite a noble metal it is not easy to etch with just an acid, it needs an oxidizer like NaPS.
A typical PCB etching solution contains:
• 1000 ml water
• about 250 g sodium peroxodisulfate
optionally you can boost the etching rate by adding a catalyst:
• 1 ml HgCl2 solution (about. 5 mg Hg) Degussa company recipe, but we don´t want mercury
• use silver ions (Ag+) instead as silver flakes or some drops of silver nitrate solution (AgNO3).
Etching process should be carried out in the temperature range of ca. 40 to 45 °C – higher temperatures lead to higher decomposition with the release of oxygen gas. About 40g/l of copper can be dissolved in the etching solution, that is all the copper on 10cm * 127cm of 35µ PCB and approx. 6 kg of sodium peroxodisulfate are needed to dissolve 1 kg of copper. The copper and sodium peroxodisulfate content in the etching solution can be determined by the usual analytical methods i.e. colourimetry.
In sum the etching reaction mechanism is: Cu + Na2S2O8 -> CuSO4 + Na2SO4
The ionic equation for this redox reaction is: Cu0 + [S2O8]2- -> Cu2+ + 2[SO4]2-+ energy
We can write this overall reaction as two half-reactions:
the copper oxidation reaction: Cu0 -> Cu2+ + 2e– with E0 = +0.340V
and the reduction reaction of peroxosulphate to sulphate: [S2O8]2- + 2e– -> 2[SO4]2- with E0 = +2.010V
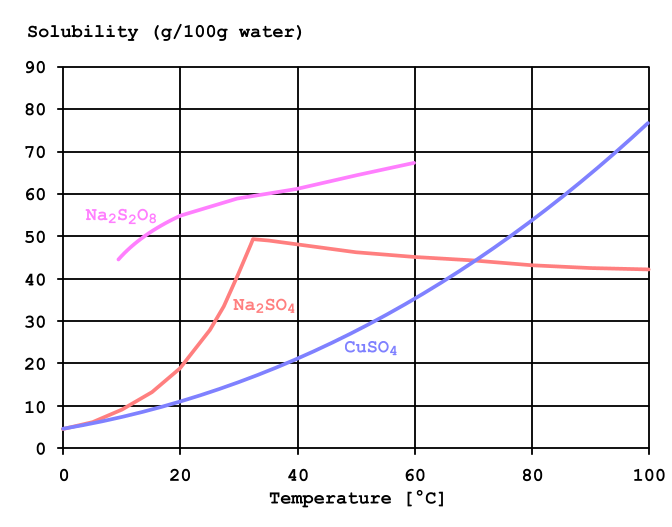
From the solubility curves of the components we can guess that the clear crystals are, which sometimes precipitate in older solutions – sodium sulfate:
Waste water treatment & Disposal of etching baths
For waste water treatment the spent etching solution should be diluted in a big container & steel-wool added.
Etching solutions containing Cu ions, which are highly toxic to fish & bacteria in the biological stages of your city´s wastewater treatment plant and must not enter drains. The copper content of the discharged wastewater must not exceed a value of 2 mg per litre. Etching baths must therefore either be disposed of as hazardous waste or be prepared so that this limit is met. Since etching baths are treated as inorganic laboratory waste, the special disposal is very expensive.
It is therefore worthwhile to reduce these costs significantly by processing the solution yourself.
Processing: Etching baths are diluted to double the volume. Fill a maximum of three quarters of a 2l bottle & add steel wool (size 00, available at hardware stores) – this reduction will happen: Cu2+ + Fe -> Cu + Fe2+ , what means we get metallic copper & iron sulfate in this reaction: Fe + Na2S2O8 -> FeSO4 + Na2SO4.
It is exothermic – so be careful not to add steel wool too fast. The solution is allowed to stand for 3 hours, adding bits of steel wool, until they no longer dissolve & the reaction is complete. During this time the colour changes from blue to the pale green of Fe(II) ions. Take some ml of the liquid & mix it with 1 ml of concentrated ammonium hydroxide (NH4OH): If no blue colour appears the copper concentration is below the permissible level for waste water discharges. If a blue color occurs, the process must be continued with more steel wool.
If you have time allow the yellowish-green solution to stand 1-2 days in an open container or by passing air to oxidize the Fe(II) ions to Fe(III) ions. This step is not really necessary. The solution can then be added to the waste water. It only contains non-toxic iron-, sodium- & sulfate ions.
Decant the solution or filter out deposited copper (eventually together with sodium sulfate crystals) then let dry and place in a sealed polyethylene bag as household waste, metallic copper is no more toxic….



